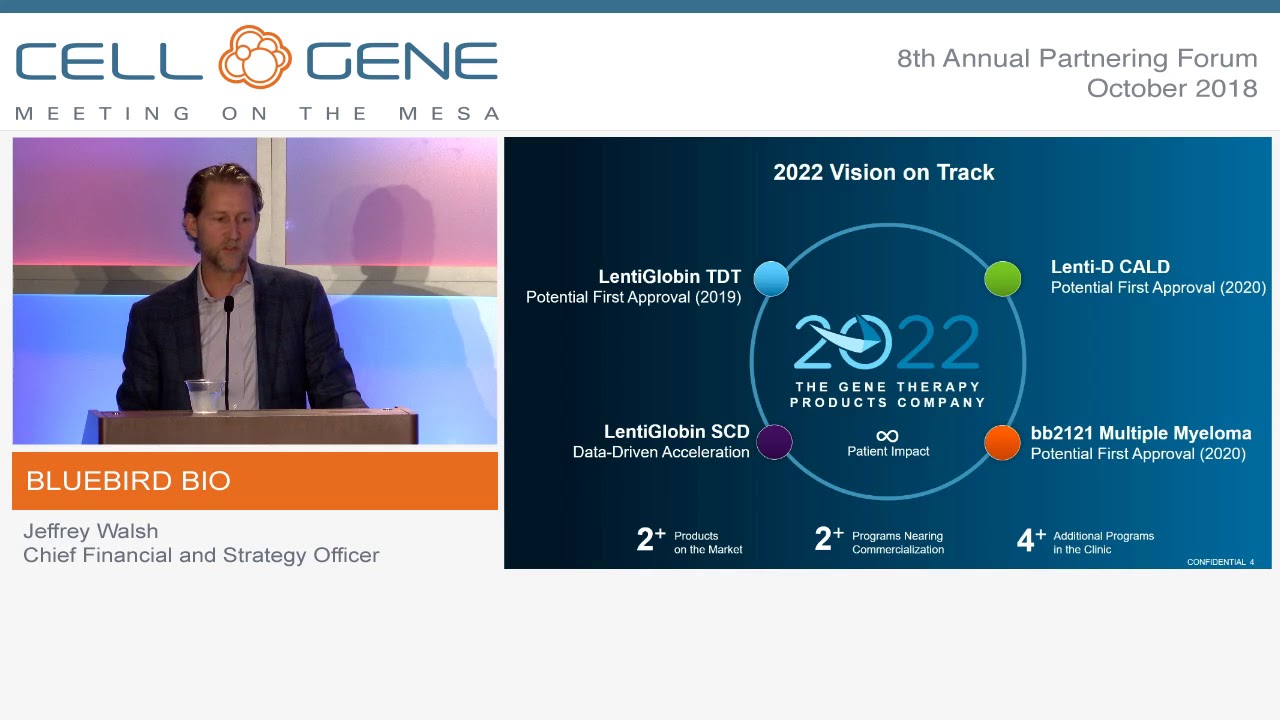
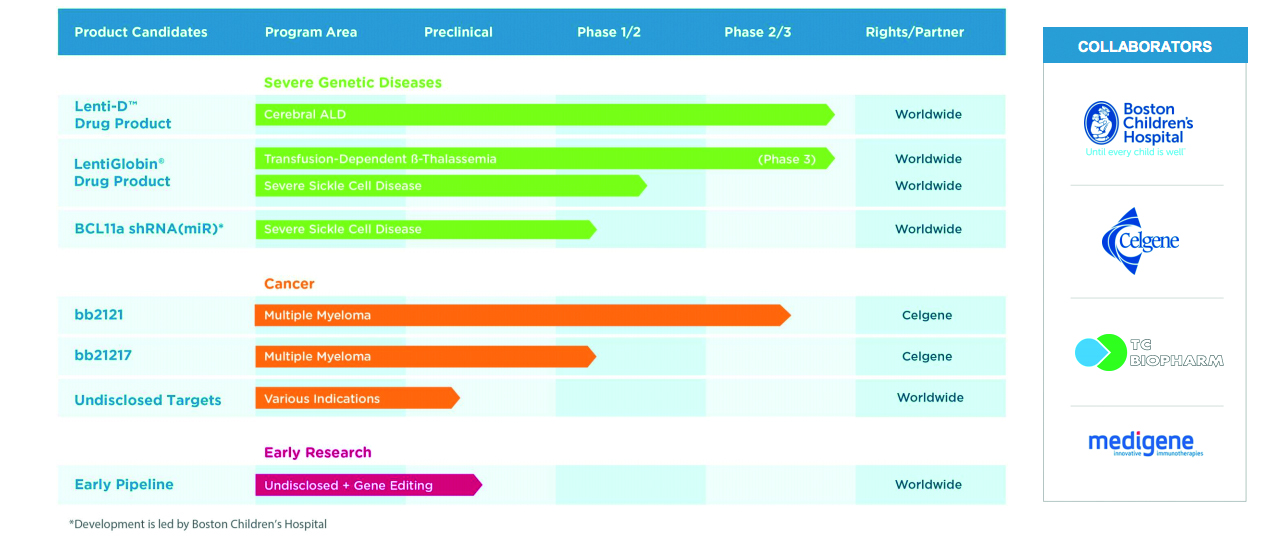
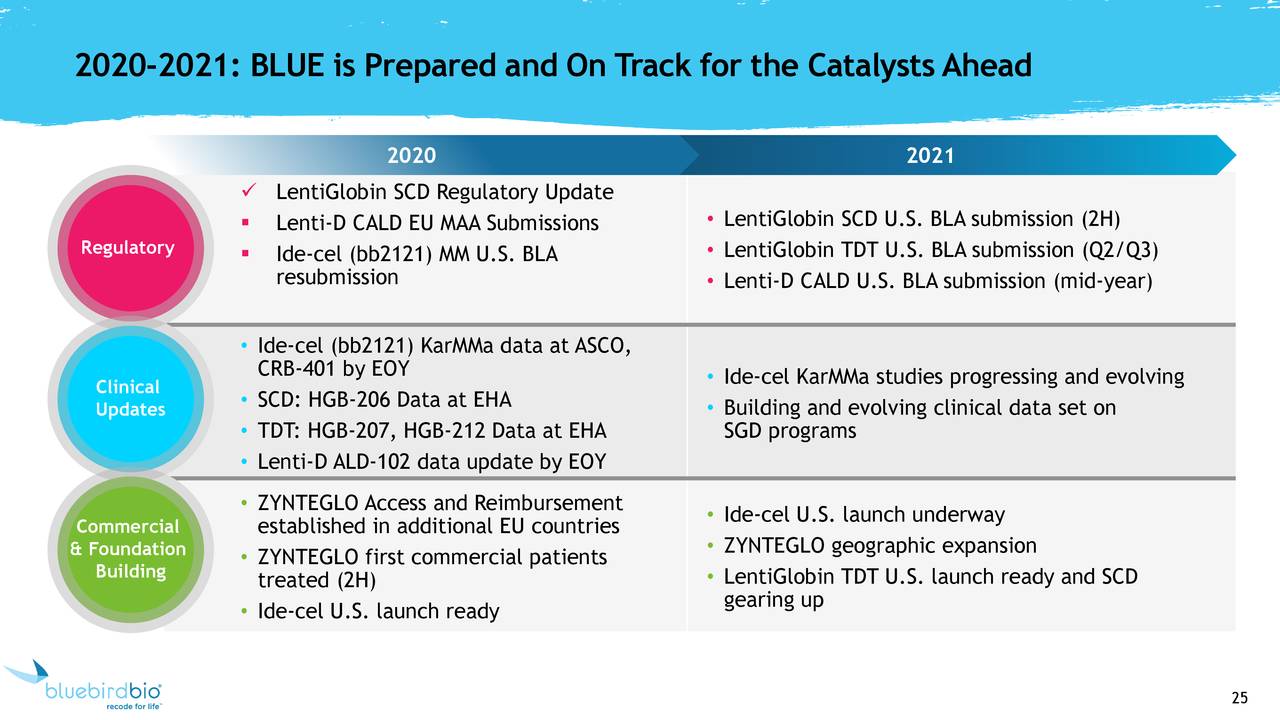
bluebird bio Receives EC Approval for SKYSONA™ (elivaldogene autotemcel, Lenti-D™) Gene Therapy for Patients Less Than 18 Years of Age With Early Cerebral Adrenoleukodystrophy (CALD) Without Matched Sibling Donor - bluebird bio,
bluebird bio: Market Fails To React To Impressive Data On Lenti-D Gene Therapy For CALD (NASDAQ:BLUE) | Seeking Alpha

bluebird bio: Market Fails To React To Impressive Data On Lenti-D Gene Therapy For CALD (NASDAQ:BLUE) | Seeking Alpha

bluebird bio: Market Fails To React To Impressive Data On Lenti-D Gene Therapy For CALD (NASDAQ:BLUE) | Seeking Alpha

bluebird bio Announces FDA Priority Review of Biologics License Application for eli-cel Gene Therapy for Cerebral Adrenoleukodystrophy (CALD) in Patients Without a Matched Sibling Donor | Business Wire

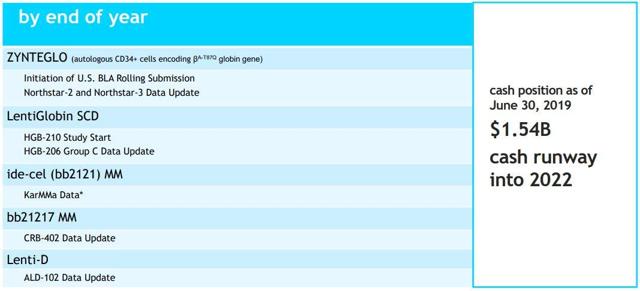
Bluebird bio Reports US FDA Acceptance of BLA for Priority Review of Lenti-D (elivaldogene autotemcel)

Bluebird Bio's Shares Drop To 52-Week Low After Another Gene Therapy Trial Placed On FDA Hold - Benzinga